A 20-year retrospective: How Kidney Cancer Journal Reflected on Advances in The Therapeutic Strategies

Robert A Figlin, MD, FACP1 , Senthil Pazhanisamy, PhD2
1. Cedars-Sinai Samuel Oschin Comprehensive Cancer Institute, Cedars-Sinai Health System, Los Angeles CA
2) Kidney Cancer Journal, Cary NC
CORRESPONDENCE: Robert A Figlin, MD, FACP
ABSTRACT
The last two decades of the kidney cancer therapeutic landscape encapsulate the most dramatic advances ever achieved in the management of localized and advanced renal cell carcinoma (aRCC). During these years, Kidney Cancer Journal also published some important pieces of research and provided coverage in the kidney cancer space. Herein, we would like to reflect on the journal’s contents that informed key advances in the treatment strategies, milestones, and management of cancer during the last two decades of the KCJ’s journey.
INTRODUCTION
There has been tremendous progress in the treatment landscape of aRCC with the expansion of the therapeutic armamentarium of targeted therapies. The vast knowledge that we have gained in the last few years, at a certain point, led to a qualitative and quantitative leap in the treatment era. Over the last two decades, KCJ disseminated and educated clinicians about groundbreaking research and translational scientific discoveries that served as a touchstone for potential treatment strategies. Our editorial contents have kept clinicians on the leading edge of the evolution in cancer therapy as well as closely reflected on advances in cancer care. The original concept of quarterly publication representing in-depth articles, future perspectives, scientific forums, timely reviews, latest breakthroughs, and conference coverages offered tantalizing previews of practice-changing research updates. In this review, we explore novel first-line treatment strategies and provide an overview of the efficacy and safety of emerging investigational
agents in the front-line aRCC setting.

VEGF-targeted therapies
The first-line treatment landscape has transitioned from recombinant cytokines to tyrosine kinase inhibitors (TKI), mammalian target of rapamycin (mTOR) inhibitors, and most recently, immune checkpoint inhibitors (ICI) in recent years. With the improved understanding of the implications of von Hippel– Lindau gene mutations in angiogenic pathways, many VEGF-based tyrosine kinase inhibitors (TKIs) evolved as the de facto choice of first-line systemic therapy1.


TABLE 1 | Summary of phase III front-line combination trials in Renal Cell Carcinoma.
For the favorable-risk disease category. Over the last few years, KCJ provided in-depth coverage on VEGF-TKI-based targeted therapies. In particular, our roundtable discussion provided expert perspectives on cabozantinib in 2015 and 20172,3. Similarly, our roundtable discussions provided coverage for tivozanib monotherapy based trials in 2021 and 20224,5 and its combinations6,7. Based on the phase III trial outcomes, sunitinib, pazopanib, and bevacizumab/IFN-α angiogenic agents8-11 were approved by FDA/ EMA as a front-line treatment. Sunitinib and pazopanib represent an effective first-line VEGFR TKIs and NCCN Kidney Cancer Panel has listed sunitinib and pazopanib as preferred category I. For more than a decade, sunitinib, an orally administered multi-target TKI remained the standard-of-care targeted therapy and as main comparator in clinical trials as well. The survival benefit of sunitinib was evident in the pivotal randomized phase III trial in which sunitinib treatment resulted in improved PFS as compared with IFN-α in the first-line setting (11.0 vs. 5.0 months)8. Although a higher OS in patients treated with sunitinib was observed as compared with those treated with IFN-α (26.4 versus 21.8 months, respectively), it lacked statistical significance8,9 . In 2006 by the FDA and EMA sunitinib was approved multi-nationally for the first- and second-line treatment of metastatic renal cell carcinoma (mRCC).
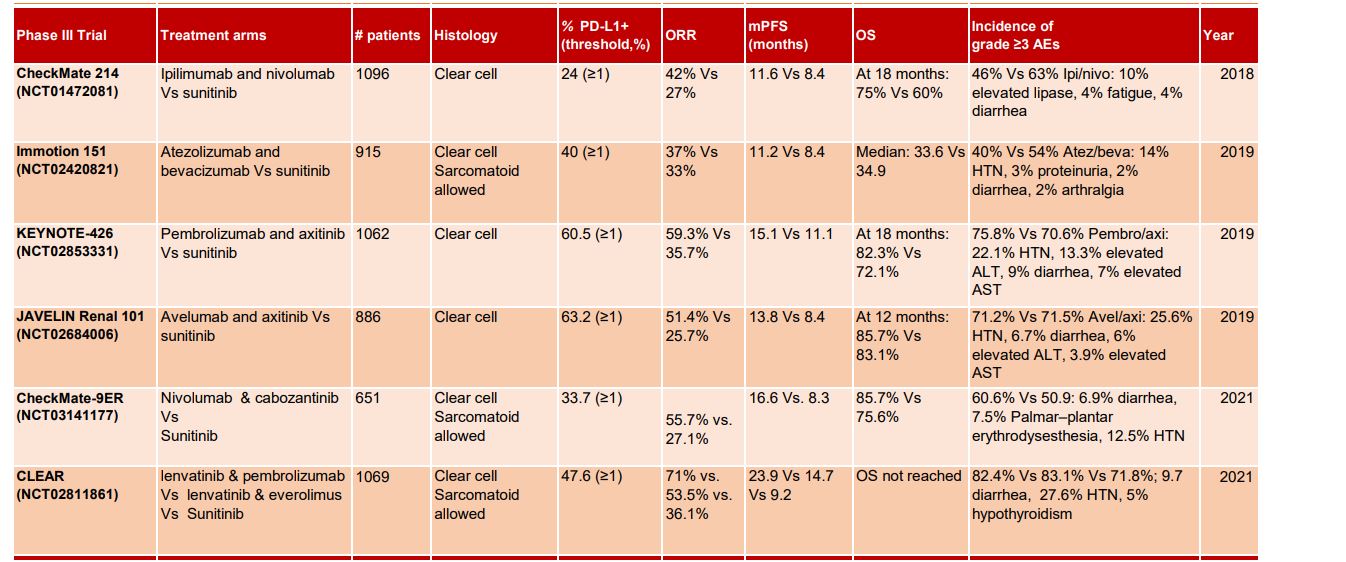

TABLE 1 | Summary of phase III front-line combination trials in Renal Cell Carcinoma.
.Based on a randomized, double-blind, phase III VEG105192 study, the FDA approved the use of pazopanib for the treatment of aRCC in 2019 and the EMA approved it for the first-line treatment of aRCC in patients who received prior cytokine therapy for advanced disease in 201010. In a phase 3 AVOREN trial of bevacizumab, a monoclonal antibody directed against the VEGF receptor (VEGFR) plus interferon-2α (IFN) showed significant improvements in PFS (10.2 vs. 5.4 months, p = 0.0001) in contrast to treatment with interferon-α monotherapy in mRCC10. Overall, this AVOREN trial confirms that bevacizumab plus IFN remains the first-line standard of care for patients with mRCC11. Multiple phase III randomized studies for eg. TARGET, COMPARZ demonstrated the survival benefits of sorafenib, pazopanib respectively10,12. Altogether these clinical trials validated the use of VEGF targeting agents the first-line standard of care for patients with mRCC.
Cabozantinib is an oral TKI that targets multiple tyrosine kinases, including hepatocyte growth factor (cMet), VEGFRs, and AXL. The randomized, phase 2 CABOSUN trial compared cabozantinib with sunitinib in treatment-naïve patients with intermediate-/poor-risk disease by IMDC. Cabozantinib therapy improved PFS (8.2 vs. 5.6 months) and ORR (46% vs. 18%) and reduced rate of progression or death as compared to sunitinib in treatment-naïve patient13,14. Following the encouraging results from the CABOSUN trial, NCCN treatment guideline included cabozantinib as a first-line treatment option for IMDC poor- and intermediate-risk patients (category 2A). Currently, cabozantinib represents a suitable targeted first-line agent, especially among patients who are not eligible to receive immunotherapy. The safety profile of cabozantinib data from the phase III METEOR study was also consistent as seen in CABOSUN, where cabozantinib therapy was associated with significantly improved PFS, OS, and ORR versus everolimus in VEGFR-TKI pretreated patients with aRCC13,14.
Tivozanib, a highly selective and potent VEGF TKI, has demonstrated single-agent efficacy with minimal off-target toxicities and a favorable adverse event (AE) profile. A randomized controlled TIVO-1 trial has shown that tivozanib, a potent VEGFR-1, VEGFR-2, and VEGFR-3 inhibitor prolongs PFS (12.7 months) as compared with sorafenib (9.1 months) in the prespecified subpopulation of treatment-naive patients15,16. Although, ORR was higher with tivozanib compared with sorafenib per independent review, the sorafenib arm had higher OS. Tivozanib treatment was associated with fewer AE-related dose reductions and dose interruptions compared with sorafanib. Due to the limited benefits from the data, tivozanib monotherapy has not been approved outside of the EU for the treatment of adult patients with relapsed or refractory advanced RCC who have received two or more prior systemic therapies. Later, revised data from the second prespecified analysis of the TIVO-3 trial indicated better survival benefits with a hazard ratio for OS of 0.99 for tivozanib compared with sorafenib15-18. These durable improvements further validated the potential for tivozanib. In KCJ, we closely covered insightful developments of specific targeting agents especially cabozantinib, and tivozanib2-5.
mTOR inhibitors
mTOR inhibitors also evolved in parallel to the development of VEGF inhibitors in the mRCC landscape. Currently, both everolimus and temsirolimus are effective mTOR agents for the treatment of aRCC. Temsirolimus, a potent mTOR inhibitor, was approved for first-line treatment of advanced RCC following the favorable outcome obtained from the multicenter, phase 3 ARCC trial (NCT00065468). Temsirolimus monotherapy as compared to temsirolimus plus IFN-α combination significantly prolonged OS compared with IFN-α19. However, superior A more pronounced survival advantage was observed only in patients with non-clear cell histology19. Given such modest results and also due to its weekly intravenous injection limitation, temsirolimus is not a widely used therapy in the front-line for patients and its utility has been relegated to second or later lines of therapy for patients with poor risk prognostic features.
Immune Checkpoint Inhibitors
In the last decade, a new avenue of immune checkpoint inhibitors has revolutionized the treatment of patients with advanced renal cell carcinoma, with the potential for dramatic changes in the therapeutic landscape. Owing to their superior and improved overall survival across multiple clinical trials, immune checkpoint-inhibitors (CPIs) such as PD-1 (anti-programmed death receptor 1), PD-L1 (anti-programmed death receptor ligand 1), and CTLA-4 (anti-cytotoxic T lymphocytes antigen 4) have been integrated into the first-line therapeutic landscape for moderate to high-risk mRCC. Since the approval of the CTLA-4 antibody ipilimumab in patients with melanoma in 2011, the footprints of ICIs expanded across the RCC landscape following studies of several PD-1/PD-L1 inhibitors and our coverages in KCJ highlighted the following progress made as well.
Nivolumab, an ICI that targets the programmed cell-death protein 1 (PD1), has become the standard treatment for patients with mRCC following progression to single-agent tyrosine kinase inhibitors (TKI)20. CheckMate-214 (NCT02231749) evaluated the CTLA-4 blocker (ipilimumab) and PD-1 inhibitor (nivolumab) combination in the IMDC intermediate or high-risk population21. The outcomes validated the proof of concept that this combination can deliver better outcomes as compared to the anti-VEGF TKI sunitinib in the first line metastatic RCC setting. Importantly, improved response rates (42%, 9% CR vs 27%, 1% CR), PFS (11.6 mo vs 8.4 mo), and OS (NR vs 26.6 mo) were observed in combination arm as compared to sunitinib. In particular, the addition of ipilimumab to nivolumab resulted in significantly better overall survival and improved ORR as compared to sunitinib. This nivolumab/ip¬ilimumab combination is considered for intermediate-risk disease for patients who cannot receive a TKI, particularly those who are younger (< 65 years) or with tumors having high PD-L1 TPS. PD-L1 expression did not predict treatment response and survival benefit was independent of PD-L1 expression21. IMmotion010 examined the utility of anti-PD-L1 atezolizumab monotherapy as adjuvant therapy in RCC patients at increased risk of recurrence after resection. Atezolizumab adjuvant therapy did not improve clinical outcomes as compare to placebo after resection in the ITT population22. Median INV-DFS was 57.2 mo for atezo and 49.5 mo (47.4, NE) for placebo. Safety analysis offered manageable profile for atezolizumab; grade 3/4 adverse events occurred in 27% (106/390) and 21% (81/383) of pts receiving atezo or placebo, respectively; Grade 5 AEs occurred in <1% (1/390) and <1% (3/383)22,23.
The 30-month follow-up of phase III KEYNOTE-564 trial showed a continued disease-free survival benefit with adjuvant pembrolizumab vs placebo in patients with clear cell renal cell carcinoma who are at increased risk of disease recurrence. Updated results support the use of adjuvant pembrolizumab monotherapy as a standard of care for participants with renal cell carcinoma with an increased risk of recurrence after nephrectomy24. At 30 months, the cumulative incidence of local recurrence was 3.8% in the pembrolizumab group vs 7.6% in the placebo group, and distant metastasis–free survival rates were 77.3% vs 68.824. Recently, results from the CheckMate 914 trial examining the role of adjuvant nivolumab and ipilimumab were presented at ESMO202225. This study did not meet the primary endpoint of DFS over a median follow-up of 37.0 months. In patients treated with nephrectomy for localized renal cell carcinoma (RCC) at a high risk of relapse, median DFS was not reached among patients who received nivolumab and ipilimumab and was 50.7 months among those who received placebo25.
ICIs in combination with VEGF-TKI
Emerging data validate the synergistic effect of ICI agents in combination with anti-VEGF targeted agents that gaining momentum as the first-line treatment landscape of aRCC. The ongoing phase 3 COSMIC-313 trial evaluates the combination of cabozantinib, nivolumab and ipilimumab versus the combination of nivolumab and ipilimumab in patients with previously untreated advanced intermediate- or poor-risk RCC. In the COSMIC-313 study26, cabozantinib was predicted to synergize with nivolumab plus ipilimumab CPI combination, as a triplet regimen for the first-line standard-of-care treatment in patients with advanced RCC of intermediate or poor risk. At a median follow-up of 20.2 months, patients who received the triplet had a 27% lower risk for progression or death compared with those receiving the checkpoint inhibitor doublet. Median progression-free survival, the primary endpoint, was not reached in the triplet group, versus 11.3 months with the doublet. Overall response rates were 43% and 36%, respectively, with complete responses achieved in 3% of patients in each group26. The disease control rates were 86% and 72%; the incidence of progressive disease as the best response was just 8% in the triplet therapy arm and 20% in the control arm. Rates of grade 3 or 4 treatment-related adverse events were higher with the TKI added, at 73% versus 41% without it. Rates of discontinuation of all treatment components were 12% with the triplet and 5% without it26.
The CLEAR trial is the latest of the IO-TKI studies examining the first-line treatment of patients with advanced clear cell RCC. The outcome data continues to show a clinically meaningful benefit from lenvatinib and pembrolizumab and reinforces this as a first-line treatment option for people with non–clear cell renal cell carcinoma (RCC) 27. After 8.2 months of follow-up, 47.6% responded to treatment with three complete responses (3.7%) and 36 partial responses (43.9%). The disease was controlled in 79.3% of patients27. In phase III, randomized keynote-426 trial (NCT03075423), treatment with pembrolizumab plus axitinib resulted in significantly longer OS and PFS, as well as a higher ORR, than treatment with sunitinib among patients with previously untreated advanced renal-cell carcinoma. After a median follow-up of 12.8 months, the combination resulted in better OS (median not reached) as compared to therapy with sunitinib (35.7 months) and superior PFS (median 15.4 vs 11.1 months)16. This study validated the benefit of pembro + axi combination therapy28. The benefit of pembro/axi was observed across all IMDC risk groups, regardless of PD-L1 expression.
In another randomized phase III JAVELIN Renal 101 (NCT02684006) trial, investigators evaluated the efficacy of axitinib and avelumab combination in treatment-naive RCC patients29. Avelumab plus axitinib therapy resulted in prolonged PFS and a significantly higher objective response rate than those who received sunitinib monotherapy. The mPFS in the combination arm was 13.8 months versus 8.4 months in sunitinib arm, and the ORR and CR rate were 55% and 4% in the combination arm versus 26% and 2% in the sunitinib arm respectively29. In CheckMate 9ER study, nivolumab plus cabozantinib combination had significant benefits over sunitinib in terms of PFS and OS in patients with treatment naïve aRCC. The mPFS was 16.6 months with nivolumab plus cabozantinib and 8.3 months with sunitinib30. The probability of OS at 12 months was 85.7% with the combination arm and 75.6% with sunitinib. An OR occurred in 55.7% of patients in the combination arm versus 27.1% in sunitinib arm (P<0.001). Efficacy benefits with nivolumab plus cabozantinib were consistent across subgroups30. In a non-randomized Phase Ib/II study, tivozanib plus nivolumab combination was assessed in patients previously treated with one oral TKI (NCT03136627). The combination of tivozanib with nivolumab prolonged disease control (median PFS of 18.9 months) and also showed a tolerable AE profile in both treatment-naïve and previously treated metastatic RCC31. The ORR was 56%, with one patient achieving a complete response.
Patients with the favorable-risk disease tend to have highly angiogenic tumours, and results from IMmotion151 support the notion of superior clinical benefits from VEGFR TKIs in this setting. The phase 3 IMmotion 151 study compared atezolizumab/bevacizumab with sunitinib32. The combination was favored over sunitinib for PFS in PD-L1+ patients. The PFS benefit was maintained in the ITT population and across subgroups of clinical interest in the PD-L1+ population, including patients with liver metastases, sarcomatoid subtype, or favorable-risk disease. Safety analysis indicated that atezolizumab/bevacizumab combination was well tolerated as patients receiving the combination had fewer treatment-related AEs relative to those receiving sunitinib (40% vs 54% for grade ¾)32. Although the combination of ICI and antiangiogenics has shown encouraging preliminary antitumor activity for advanced or mRCC, a high incidence of toxicity along with a less favorable tolerability profile may compromise the benefits in patients. For instance, in the phase I study CheckMate 016 (NCT01472081), the efficacy and safety of nivolumab in combination with antiangiogenic tyrosine kinase inhibitors or ipilimumab for the treatment of mRCC. The addition of sunitinib or pazopanib to nivolumab resulted in a high incidence of high-grade toxicities, limiting its scope in future trials33. Given the possibility that long-term cumulative adverse effects from the antiangiogenic and ICI combination may accumulate over time to outweigh the benefits, such combinatorial therapies warrant close monitoring to avoid unprecedented risks. In phase 3 PIVOT-09 trial, investigators sought to evaluate the combination efficacy of bempegaldesleukin plus nivolumab compared to sunitinib or cabozantinib as the first-line therapy for advanced renal cell carcinoma (RCC) 34. This combination did not improve outcomes vs the investigator’s choice of TKI in first line treatment of advanced/metastatic clear-cell RCC. Among patients with intermediate or poor risk disease, the ORR was 23.0% for combination arm vs 30.6% for the TKI arm. The complete response rates and clinical benefit rates were higher in the TKI arm. However, among responders, the duration appeared somewhat longer in the combination arm34.
Other novel approaches
Belzutifan, a highly selective hypoxia-inducible factor inhibitor (HIF-2α), offers a novel approach, taking a different path than commonly used to treat RCC. Most recently, the open-label study 004 (NCT03401788) has validated the efficacy and safety of belzutifan in patients with VHL-associated RCC35. Treatment with belzutifan resulted in an ORR of 49%. Based on these data, FDA approved belzutifan for adult patients with von Hippel-Lindau (VHL) disease who require therapy for RCC and other tumors36. The LITESPARK-003 trial (NCT03634540) evaluated the synergistic effect of adding belzutifan to cabozantanib therapy in patients with aRCC who previously received immunotherapy at 24.6 months of follow-up37. Results showed that the overall ORR in the intention-to-treat population (N = 52) was 31%. The ORR was 27% and 32% among patients with favorable-risk disease (n = 11), and intermediate/poor-risk disease (n = 41) respectively37. Trial recruitment is underway for the phase 3 LITESPARK-011 trial (NCT04586231) assessing belzutifan plus lenvatinib vs cabozantinib in patients who previously had anti–PD-1/PD-L1 therapy.
Surgical management
Even in the era of targeted therapy, we have been continuously providing coverages on the latest updates in the surgical management of kidney cancer, including a recent article in KCJ that reported the Latinx disparity in surgical approach for kidney cancer. Despite revolutionary advances in targeted systemic therapies, durable responses remain rare. Currently, CN is the only opportunity for the cure at an early stage. Therefore, until systemic agents provide significant curative impact, surgical resection will remain the benchmark for a long-term cure. On the other hand, despite the curative impact of surgical resection, it is estimated that nearly 30% of the patients will experience a relapse of renal cancer. Whereas, the role of CN and metastasectomy of local recurrence in advanced RCC remains unclear in the era of targeted therapies. In a Phase III PROSPER (ECOG-ACRIN EA8143) study38, investigators compared perioperative nivolumab versus observation in patients undergoing nephrectomy alone. RFS was similar between the arms. The median RFS was not reached. OS was not mature at the time of analysis but was not statistically different between study arms. Similar withdrawal rates occurred in both arms, approximately 12% (48/404 patients in nivo arm vs. 50/415 in the surgery alone arm). 20% of patients treated with nivo experienced at least one Grade 3-4 AE that could be attributable to nivo, compared with 6% in the control arm38.
CONCLUDING REMARKS
The frontline treatment paradigm in renal cell carcinoma continues to evolve, with the advent of novel ICI or ICI/TKI combinatorial regimens as reflected in the coverages published in the KCJ over the few years. In the future, a deeper understanding of immune checkpoint biology might reveal new therapeutic targets beyond PD-1, PD-L1, and CTLA-4, as well as new combination approaches. However, controversies remain regarding the precise treatment selection, sequencing, and individualized therapeutic strategy, thanks to unmet clinical need of identifying reliable predictive markers of response to immune agents and absence of head-to-head comparison among the randomized trials. Currently available approaches viz. PD-L1 expression, gene expression signatures, CD8+ T cell density cannot still predict treatment response to ICIs and/or TKIs. Importantly, validated biomarkers are essential to match patients to single-agent treatment with TKIs or immunotherapy, or combinations of immunotherapies with TKIs or novel agents. As novel treatments come to the clinic, there is a need to develop strategies for sequencing new and established therapies. Once optimized, such strategies will deliver robust survival outcomes while preserving the quality of life and as well the ability to tailor therapy to the individual patient.